Atomistic Simulation Tutorial¶
This tutorial is an online learning tool designed to provide the knowledge necessary to proceed with materials development using atomistic simulation. It aims the reader to obtrain practical skill at the code level.
Target Audience¶
The target audience is as follows
A specialist in atomistic simulation and would like to learn more about calculations of other fields.
Experts in science who do not specialize in atomistic simulations (e.g. materials informatics expert) and who would like to deepen their understanding and use of atomistic simulations in the future.
Experimentalists who would like to perform calculations to explain the results of their experiments from a theoretical perspective.
The code in this tutorial mainly runs on the Matlantis system. You will need to subscribe to the product to actually run it, but you can still learn by just reading through it. We hope that you will be able to advance to the level of understanding the various examples of calculations and papers published below by completing this tutorial.
Matlantis case studies and papers: https://matlantis.com/ja/cases/
Matlantis contrib repository:https://github.com/matlantis-pfcc/matlantis-contrib
Scope of this tutorial¶
Materials¶
A variety of materials exist, from organic molecules to metals and semiconductors. We focus on industrially used solid materials currently. Material areas include, for example, the following:
Catalysts
Battery Materials
Metals
Semiconductors
Ceramics
Organic molecules
Lubricants
Polymers, etc.
Molecules that act on living organisms are out of scope at this time because of the large number of atoms involved and the need for a different approach.
Simulation Scale¶
There are many methods for computational materials development, each of which deals with different spatial and temporal scales.
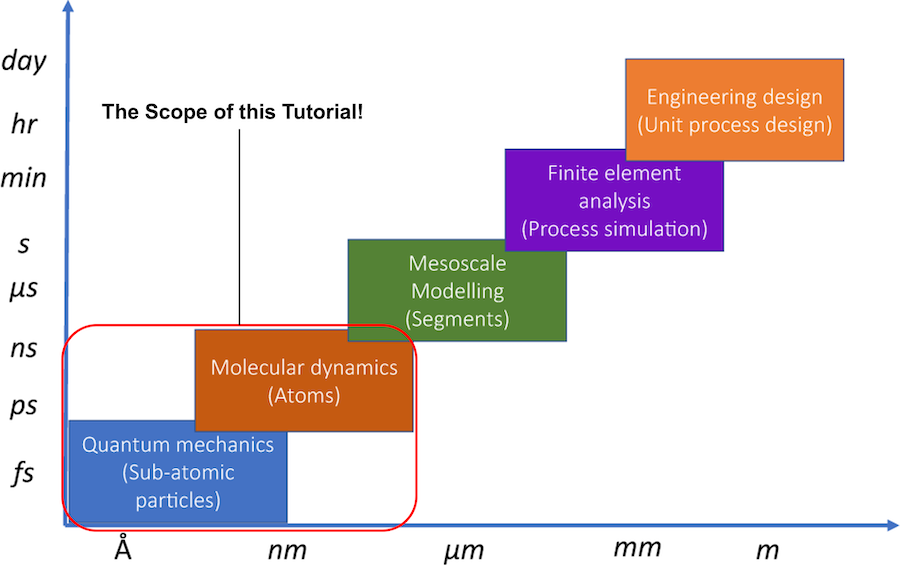
Created by modifying Fig. 1: Length and time-scale based computational materials design techniques from “The joint automated repository for various integrated simulations (JARVIS) for data-driven materials design” (Licensed under CC BY 4.0)
This tutorial focuses mainly on the atomic scale in the above figure, and covers problems that are analyzed by conventional quantum chemical calculation methods (e.g., DFT) and molecular dynamics. However, we focus on “atomistic simulation” that deals with phenomena at the atomic level, and do not include microscopic analysis such as electronic structure analysis (e.g., band gap analysis of semiconductors).
Matlantis¶
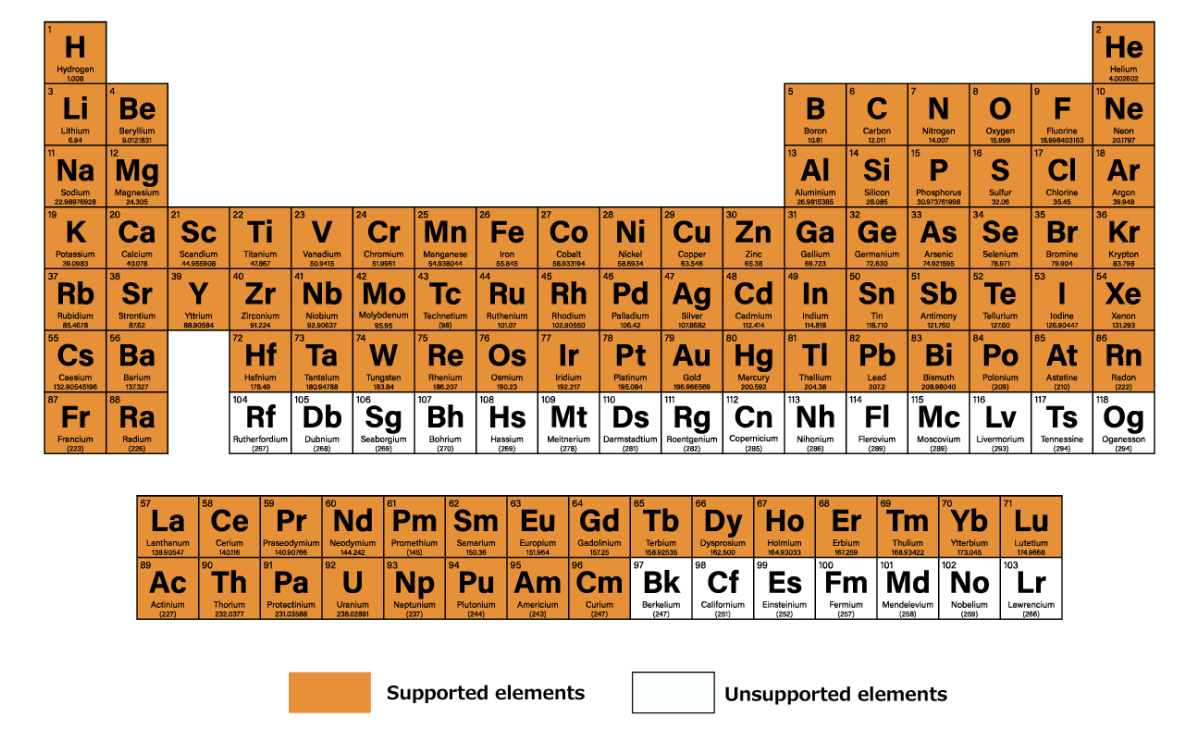
The code in this Tutorial can be run on Matlantis systems. Matlantis is a universal atomistic simulator provided by Matlantis Corp., whose mission is “to accelerate materials discovery for a sustainable future”.
Versatility
Speed
User-friendliness
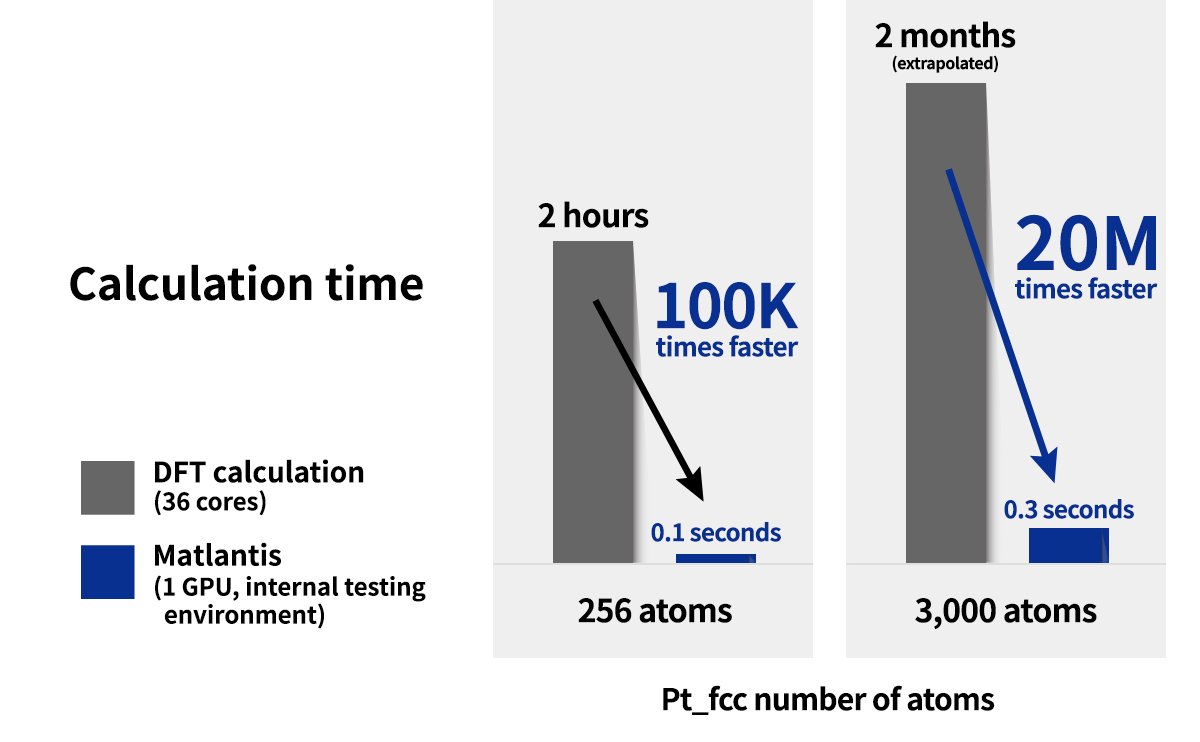
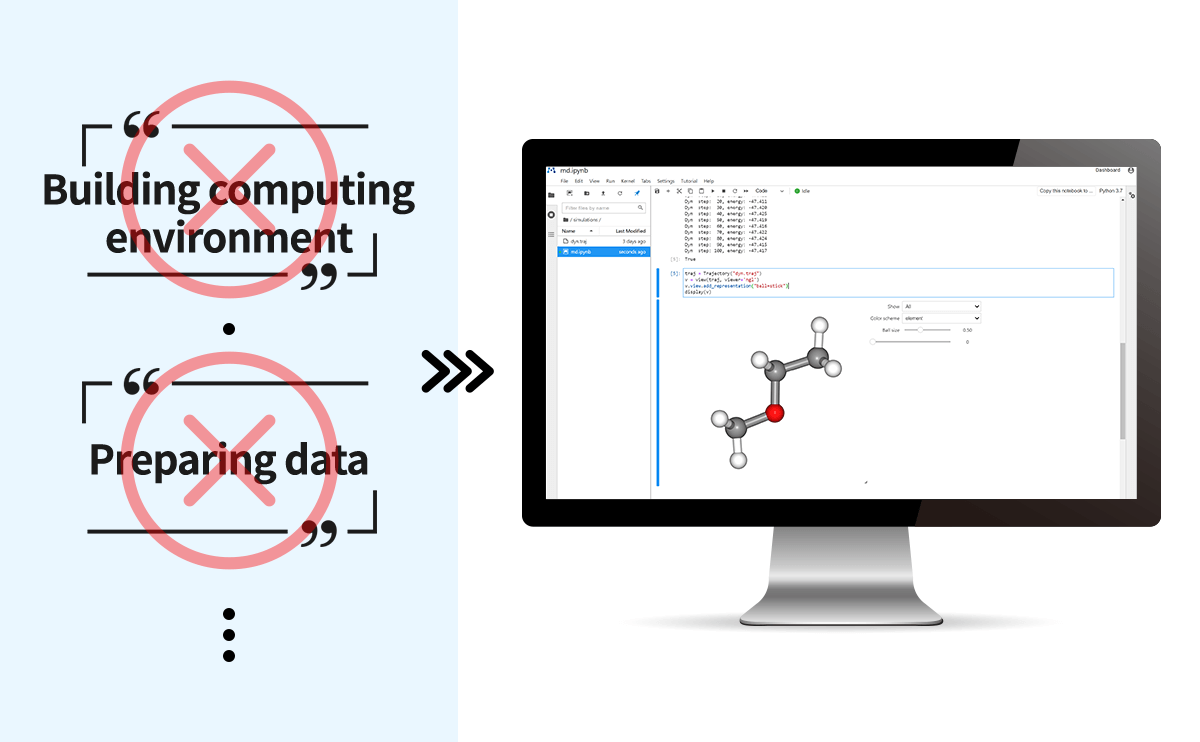
It offers the potential for versatility and can be used for a wide range of elements and structures. Compared to quantum chemical calculations it is faster. It is also easier to use, with the interactive execution on the Jupyter Lab.
These characteristics are also suitable for learning by trial-and-error, as results can be obtained immediately. For more information on the core technologies used in the Matlantis, please refer to the following references.
Why do we learn atomistic simulation?¶
Atomistic simulation can be used to elucidate the mechanisms of experimentally known phenomena and to develop new materials by analyzing elemental combinations and structures that have never been expected before. In recent years, the word “SDGs (Sustainable Development Goals)” has become more popular. SDGs are not only about making the world more convenient, but also about making the world better in a sustainable manner. Let us give some concrete examples of how materials development through atomistic simulations can be used to approach the realization of a sustainable world.
Ammonia synthesis catalyst¶
At the beginning of the 20th century, when the explosive growth of the world’s population led to a food crisis, increasing grain productivity was an urgent matter. There are three essentials needed for grain production: water, sunlight, and good quality soil. Since nutrients in the soil are sucked up by plants, a good fertilizer is needed for a consistent annual harvest.
Fertilizers require a nitrogen component, but artificial fertilizers such as manure have been used at that time, making mass production difficult. Under these social conditions, the Haber-Bosch process, which synthesizes ammonia (NH3) from nitrogen (N2) in the air by using iron as a catalyst, was established, making it possible to produce synthetic fertilizers. In other words, the Haber-Bosch process made it possible to “make bread from air” and industrial production of food became possible.
This method is developed in 1906 and is still used in various industrial processes more than a century later, with the base catalytic material remaining the same. However it is a method that consumes large amounts of energy. It has been reported that 2% of the world’s energy consumption is used for the Haber-Bosch process. If a catalyst can be found that improves the efficiency of the reaction, the effect will be very large.
Reference
Battery materials¶
The demand for energy storage is increasing rapidly for smartphones, computers, electric vehicles, and household storage batteries etc. When charging and discharging a lithium-ion battery, lithium (Li) moves in and out of the electrolyte. So, how fast Li can move within the battery is important. The all-solid-state lithium-ion battery is one of the most promising developments in the storage battery market because of its high level of safety, however, it is difficult to conduct Li ions at high speed, which is a barrier to their full-scale mass production, and it is important to develop a solid electrolyte with high ion conductivity.
In the following calculation example, the diffusion coefficient of Li ions is obtained by MD (Molecular Dynamics) simulation at the atomic level. It shows atomistic simulation can be useful for the development of solid electrolyte materials.
Reference
Hydrogen and synthetic fuel generation catalysts for renewable energy storage¶
The use of renewable energy is increasing as part of efforts to reduce CO2 in the world. Solar power generation is limited to sunny days and during the daytime, and wind power generation is limited to the time when the wind is blowing. Storage of this energy will be necessary for further expansion of its use in the future.
It is estimated that battery storage alone is not large enough in scale to store the energy on a large scale. Energy storage by converting to hydrogen or methane, which can be stored on a large scale using existing facilities, is considered promising. Currently, the challenge is to find a catalyst that can achieve highly efficient conversion of these reactions:
Making hydrogen (H2) and oxygen (O2) from water (H2O)
Produce synthetic fuels such as methane (CH4) from hydrogen (H2) and carbon dioxide (CO2)
If a catalyst that can increase the efficiency of such reactions can be found, it is expected to be possible to store energy obtained from renewable energy and use it at another time and place.
The following references provide more detailed explanations.
References
We will introduce reaction analysis on catalysts in chapter 5, and molecular dynamics to calculate Li-ion diffusion in chapter 6.
Of course there are many other applications, including semiconductor, lubiricant, MOF adsorbent.
We would be happy if you could perform simulations on a variety of materials and contribute to the discovery of new and innovative materials.
Learning how to use Python and some libraries¶
In this tutorial, we will use Python programming, and code is executed on Jupyter Lab. If you have no programming experience before, or if you are not comfortable using NumPy, a major library commonly used in Python, you can learn Chainer Tutorial’s 1. ~ 12. (sorry this is Japanese only).
You may refer other online contents to learn basics of python and its commonly used library, for example
Some reference might be written in Japanese, in that case translation tools such as DeepL or Google translate can be used.
Let’s start learning atomistic simulation by running the code in the next section.